 Medical Device Design – How & Where Design Mark Fits In
Medical Device Design – How & Where Design Mark Fits In
Medical device design involves extensive, overlapping collaboration between Design Mark and the client. It entails analyzing multiple factors at once while efficiently moving product development forward in the most cost effective way possible. Design Mark is an ISO-certified, solutions-based company with over 45 years of experience in navigating the entire medical device design program on behalf of our clients. From concept development to prototype analysis, we advise and execute design solutions that meet the highest standards possible.
Intuitive, Highly Disciplined Medical Device Design
The design and manufacturing of medical devices is an extremely complex endeavor that requires perpetual communication between all teams involved. Overlooked factors such as compliance needs, patient ergonomics and design for manufacturing failures can never be passed as trivial mistakes in the medical field. They not only have the potential to halt product releases in their tracks, but also collapse company reputations overnight. A user-centric approach is always necessary during the creation of virtually any medical device, carefully managed by our engineers along each phase and intensively examined for cost, regulatory compliance, health and manufacturing ramifications.
For Design Mark, being consultative with our client along every step of the medical design process is absolutely necessary in order to succeed in understanding their application needs. Our engineering team is cross-functional, enabling designs that represent the best interface between the user and their equipment.
 Medical Device Concept Exploration & Market Needs
Medical Device Concept Exploration & Market Needs
Whether a device is presented to us as a new concept or if we’re tasked with developing one on our own, Design Mark can help clients envision medical device advancements or solve existing healthcare dilemmas. We can identify untapped market needs as well as leverage our specialists to innovate existing concepts while keeping tabs on the latest medical engineering trends.
Healthcare Regulatory Analysis & Device Classification Studies
As an ISO 9001:2015 certified organization, Design Mark is well versed in systematically bringing ideas in-line with current national and international medical device regulations. Our concepts are developed in conjunction with the latest medical device standards, merging intuitive engineering design within the regulatory framework of the new product’s market.
Early Stage Medical Design, Prototyping & Engineering Support
Early medical device concepts require first stage prototypes that need to be supported by highly competent, multi-disciplined engineering team. We ensure that early prototype development stays focused on user proof-of-concepts, manufacturability and cost efficiency.
Establish a Design Framework in Conjunction with Compliance Needs
Every medical device design requires a unique design framework to work under. In this stage, Design Mark will establish the necessary structure for which our designs are developed in accordance to FDA, ANSI, cGMP and other prevalent standards.
Create a Medical Device Design Risk Management Strategy
Our team integrates risk management standards in order to identify potential issues, hazards, and liabilities involved with the production of a medical device. An extensive, ongoing review process is put into place at every stage of development, including the implementation of potential failure studies (FMEA), trace-ability tests and documentation processes.
 Initiate Quality Assurance Program
Initiate Quality Assurance Program
All aspects of the medical device design process, from concept to storage, requires a quality assurance system that studies the product’s effectiveness throughout its life-cycle. This includes ensuring that all parties involved such as partners, suppliers and other 3rd party entities adhere to the device’s QA requirements. We always ensure that the device operates as intended, from production to warehousing.
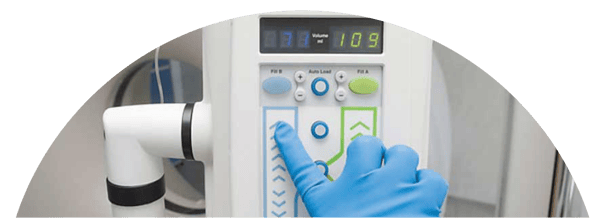
Design Mark’s User Interface (UI) Solutions
Design Mark’s medical devices are a culmination of extensive research, analysis and engineering expertise. Many of our core products include custom membrane switches and graphic overlays, all of which have undergone the same intensive medical device design process mentioned above. We’ve worked with clients across multiple healthcare markets to advise, design and manufacturer UI solutions according to their exact requirements.
Our UI products are a direct result of our design methodology, resulting in custom engineered devices that meet client, regulatory and user standards. During our design process, we’ve helped augment ideas into reality for both our membrane switches and graphic overlays used in various X-ray machines, imaging systems, handheld devices, incubators and other instrumentation. Used every day by medical facilities nationwide, our UI solutions are the result of a highly organized product development strategy.
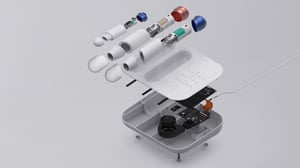
 Membrane Switches
Membrane Switches
During the medical device design process, we’ve ensured that membrane layers are not only highly sterile, but also easily sanitized and free from foreign or micro-bacterial contaminates. Lightweight, durable and tactile, our membranes have been developed to act as gaskets which maintain and protect against industrial cleaning agents. They ensure a proper seal between buttons and electronic components. Hospitals and other healthcare facilities are stocked with equipment that emit various ambient radio signals, discharges and more, all of which are carefully considered during the design and manufacturing process.
Graphic Overlays
We apply the latest ergonomic design concepts to all of our graphic overlays, blending aesthetic visuals with straightforward, uncomplicated control features. User friendly designs are a core component to any UI, providing seamless interaction for users of any skill level. We help anticipate what type of functionality these users require, so that they can easily access, understand and operate their equipment efficiently. These highly visible graphic overlays help exemplify indicators, warning lights and LED signals so that healthcare specialists can effortlessly view them from virtually any angle or noticeable distance.
In other projects, we’ve incorporated graphic overlays with advanced shielding that protects devices from electro-static discharge, electro-magnetic interference, and radio frequency signals – all of which are prevalent in technically advanced healthcare environments.
From portable devices with touchscreens to complex MRI control panels, our UI solutions take into account materials, shape, durability, sterility and instrumentation geometry. All of our medical device products are designed and manufactured in accordance to the latest RoHS compliance needs.
Good Design Practices are the Foundation to Medical Device Success
Design Mark continues to implement rigorous parameters that carefully coordinate and fuse innovation, regulation and healthcare profitability into a successful product. By implementing astute medical device development methods, we’ve helped countless organizations maintain a level of quality that surpasses today’s regulatory standards without cutting corners. However, our methods will always fall back onto our most important design goals: clear, easy to use and vibrant UI designs that offer both users and providers peace of mind.
Are you in the concept design phase and looking to get your next project started? Contact our skilled team of engineers today!












